NUCDF
INDUSTRY ALLIANCE
The landscape for the treatment of urea cycle disorders (UCDs) is rapidly evolving, thanks in large part to significant investment from our industry partners. This investment has provided the community with a wide range of treatment and therapy options. NUCDF’s unwavering mission is to save lives and improve outcomes for individuals with urea cycle disorders. In line with this mission, NUCDF will establish a transparent industry alliance program that is dedicated to delivering crucial updates on clinical trials and emerging treatments for our community. By providing the latest status updates and education materials from the industry, NUCDF families will have the essential information needed to empower them to make well-informed treatment decisions with confidence.
We invite you to join our program to help educate our community.
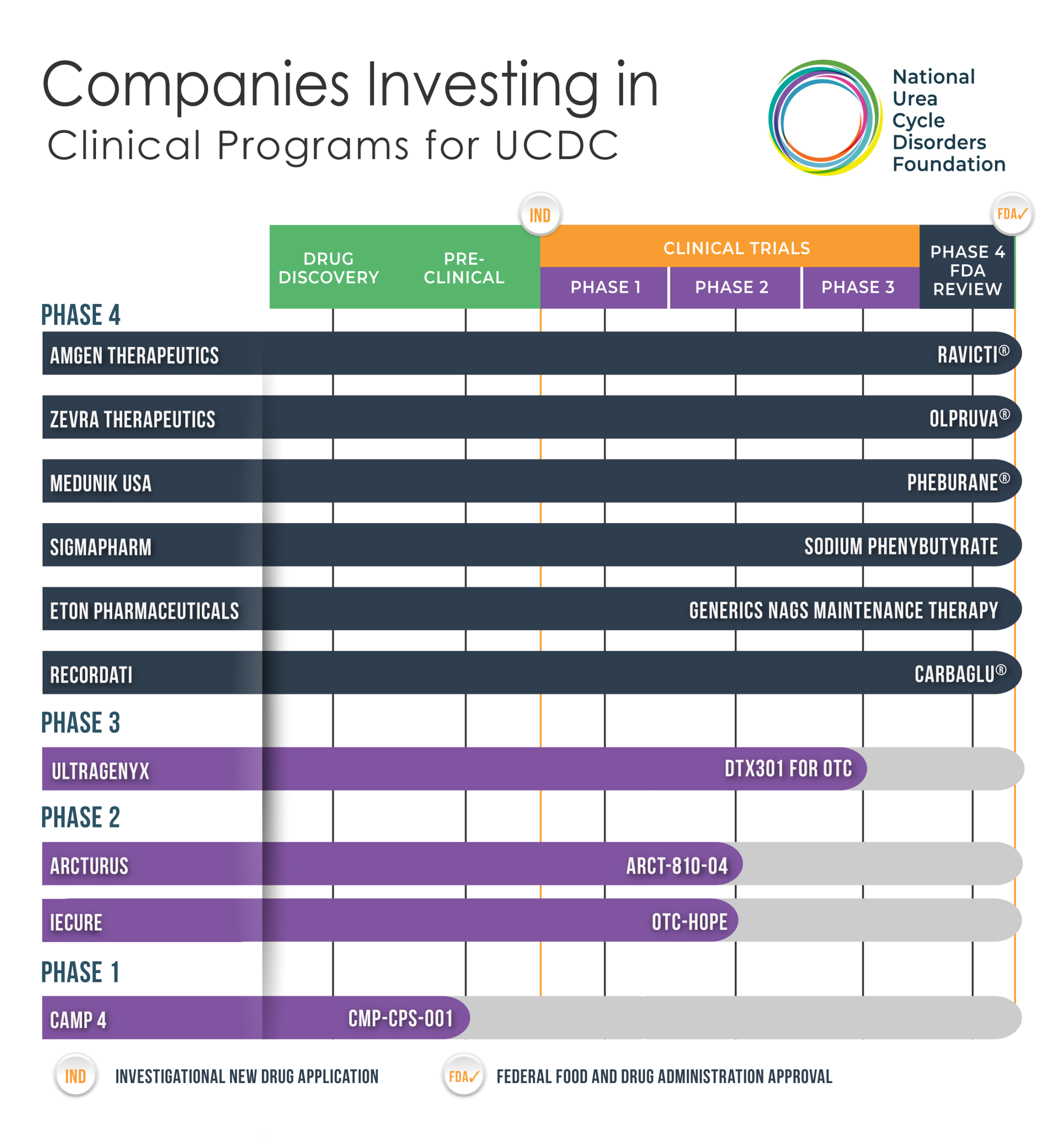
Urea Cycle Disorders Drug/Treatment Pipeline
The development of a new therapy for urea cycle disorders must follow the path that regulatory bodies have established. This path follows five structured stages spanning pre-clinical and clinical research and can take 10-25 years to complete. From early discovery to post-market safety monitoring, each stage contributes vital data that is reviewed vigorously to help ensure a new therapy is safe and effective.
04
Phase 4: Drug Review & Approval
Once a therapy has been approved by the U.S. Food and Drug Administration (FDA), it becomes commercially available in the United States. Phase 4 clinical trials are conducted after a therapy has been approved and marketed. Many of these studies are designed to continue to monitor safety and effectiveness of a newly approved therapy in the general population and to collect information about any adverse effects associated with widespread use.
AMGEN THERAPEUTICS / RAVICTI
ZEVRA THERAPEUTICS (OLPRUVA)
MEDUNIK USA (PHEBURANE)

Product Description:
Pheburane® (sodium phenylbutyrate) is FDA-approved as a safe, effective and PALATABLE treatment option for certain Urea Cycle Disorders (UCDs) used along with a specific diet.
Episodes of sudden, rapid increase of ammonia in the blood (acute hyperammonemia) may happen in people during treatment with Pheburane®. Pheburane® is not used for the treatment of acute hyperammonemia, which can be life-threatening and requires emergency medical treatment.
Pheburane® is a proven treatment for UCDs and can be conveniently taken in 2 ways with no mixing required.
The most common side effects include absent or irregular menstrual periods, decreased appetite, body odor, bad taste or avoiding foods that you ate prior to getting sick (taste aversion).
Please see the Full Prescribing Information (downloadable PDF) for more Important Safety Information.
Patient Resources:
Patient Website: https://pheburane.com/
YouTube Channel: Pheburane oral pellets (sodium phenylbutyrate) – YouTube
Videos:
- What is Pheburane® (sodium phenylbutyrate)?
- Demonstration Video for Pheburane (sodium phenylbutyrate)
- How to Take Pheburane (sodium phenylbutyrate)
Patient Materials (downloadable PDFs):
- Patient Brochure
- How to Take Guide
- Patient Resources
- Patient Support Enrollment Form
- Patient Information
- Instructions for Use
Patient Support Program: https://pheburane.com/patient-support
Medunik USA offers the Unik Support Program – designed to support each unique patient.
By enrolling in the Unik Support Program, Pheburane® patients will gain access to a variety of specialized support programs, including:
- Clinical Coordinator Outreach Program
- Copay/Patient Assistance Programs
- Mail Order Pharmacy Services
- Quick Start & Bridge Programs

Company Profile:
Medunik USA is a pharmaceutical company seeking to improve the lives of patients with rare diseases by providing access to specialized treatments, including for urea cycle disorders. We are committed to addressing the unmet medical needs of the rare disease community through innovation, collaboration, and compassionate care.
SIGMAPHARM (GENERIC SODIUM PHENYBUTYRATE)
eTon Pharmaceuticals (NAGS MAINTENANCE therapy)
RECORDATI (CARBAGLU)
03
Phase 3: Active Clinical Trials & Research
This phase primarily consists of testing the final dosage in real world medical practice on its usability and effectiveness in a large group of patients. Safety in the short and longer terms (1-2 yrs) are observed. Study Duration: ~2 to 4 years
Ultragenyx (DTX301 for OTC AAV8 gene therapy)
02
Phase 2: Pre-Clinical Research & Development
The goal of Phase 2 is to test effectiveness (how well the drug works) and to further evaluate safety in a larger group of people who have the disease or condition the drug is intended to treat. Study duration is ~1-2 years.
Arcturus (ART-810-04)
iECURE / OTC-HOPE (gene editing therapy)
About iECURE
iECURE is a clinical-stage biotechnology company focused on developing new therapies for rare genetic diseases, particularly those that appear early in life and currently have limited or no treatment options. Our mission is to go beyond treating symptoms by using cutting-edge gene editing technology to address the underlying genetic cause of disease. Our lead program is focused on a potential gene editing therapy for ornithine transcarbamylase (OTC) deficiency, a life-threatening inherited disorder.
Our experienced team brings deep expertise in global orphan drug development, gene therapy clinical trials, metabolic disease clinical management and the successful commercialization of rare disease therapies. We aim to leverage this expertise to guide our research and development strategy—carefully selecting the most appropriate targets and therapeutic approaches to address inherited liver diseases. At iECURE, we are committed to building a healthier future for patients and families living with these devastating conditions.
About the Name
Pronounced “ee-ah-cure,” our name is inspired by the Latin word iecur, which means liver—the organ where many critical metabolic pathways occur. iECURE’s investigational therapies are designed to address diseases caused by inborn errors of metabolism that originate in the liver. Our pipeline includes programs targeting these disorders, with one now in clinical trials.
Our Gene Therapy Approach
iECURE is advancing an investigational gene editing method known as gene insertion, which is designed to treat disease at its source—within the DNA itself. This approach places a functioning copy of a gene into a precise location in a person’s DNA to help the body begin producing the missing or malfunctioning enzyme or protein. In the case of OTC deficiency, the goal is to enable the body to make the OTC enzyme, which plays a key role in removing excess ammonia. If successful, this therapy could help restore a broken metabolic pathway, offering a meaningful and lasting benefit for affected infants and their families.
The OTC-HOPE Clinical Trial
OTC-HOPE is a Phase 1/2 first-in-human clinical trial evaluating the safety and potential benefits of iECURE’s investigational gene editing therapy, ECUR-506, in male infants under nine months old with genetically confirmed neonatal onset OTC deficiency. This is an open-label study, which means that doctors, researchers, and caregivers all know which treatment the participant is receiving. The goal is to better understand the potential of ECUR-506 as a treatment for this rare and severe disorder by monitoring its safety, and how the body responds to it.
iECURE has received regulatory clearance to conduct the study in the United States, United Kingdom, Spain, and Australia as of April 2025.
Helpful Links
For more information on the OTC-HOPE study and participating sites, visit:
- gov (NCT06255782)
- ISRCTN Registry (ISRCTN10957794)
- OTC-HOPE study website
- iECURE corporate website
Latest News
Stay informed about iECURE’s progress and clinical milestones:
- iECURE reports complete clinical response in first infant dosed with ECUR-506
- More updates: www.iecure.com/news
Questions?
We welcome outreach from patient advocacy organizations, families, and caregivers. If you have questions or would like to speak with a member of our team, please email us at: advocacy@iecure.com. Please do not leave any personal or private health information in your message.
If your doctor or a member of your care team has questions, please have them email our Medical Affairs team at medinfo@iecure.com.
01
Phase 1: Drug Discovery & Development
An experimental drug or treatment is tested in a small group of healthy volunteers (~20-100) to evaluate its safety, determine a safe dosage range, and identify side effects. Study duration is usually several months.
Advancing Research
Urea Cycle Disorders Consortium
In the past twenty years, there has been a dramatic increase in much-needed urea cycle disorder research, particularly with ongoing studies conducted by the Urea Cycle Disorders Consortium (UCDC) as part of the National Institutes of Health Office of Rare Diseases Clinical Research Network (RDCRN).
The Urea Cycle Disorders Consortium (UCDC) is a pioneering research collaborative that brings together top researchers, clinicians, and other healthcare professionals to work collectively and improve the lives of patients with urea cycle disorders.
The purpose of the UCDC is to conduct cutting-edge research that enhances the understanding of UCD and accelerates the development of new treatments. The UCDC comprises teams of doctors, nurses, research coordinators, and research laboratories at 16 academic centers in the US, Canada, and Europe, working together in close collaboration with the National Urea Cycle Disorders Foundation. One of the key roles the Foundation plays is ensuring the needs and wishes of UCD patients and families are met as we strive to improve their lives and learn more about preventing the effects of urea cycle disorders.
As a partner in the UCD Consortium, one of the key roles of the National Urea Cycle Disorders Foundation (NUCDF) is to provide critical input regarding the need for and development of new studies. NUCDF’s unique perspective as the center of the UCD community offers insight to researchers about treatment and management issues, as well as a focus for key research priorities. NUCDF also provides information to our families and affected individuals about research studies and how they can participate in advancing critical research.



